Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule. Log Octanol-Water Partition Coef SRC.
Ccl2h2 Lewis Structure - If you're searching for picture and video information related to the keyword you have come to visit the right blog. Our site provides you with suggestions for seeing the highest quality video and image content, hunt and locate more informative video content and graphics that fit your interests. includes one of thousands of video collections from several sources, particularly Youtube, therefore we recommend this movie that you see. You can also bring about supporting this website by sharing videos and graphics that you enjoy on this site on your social networking accounts like Facebook and Instagram or tell your closest friends share your experiences about the simplicity of access to downloads and the information that you get on this website. This site is for them to visit this website.
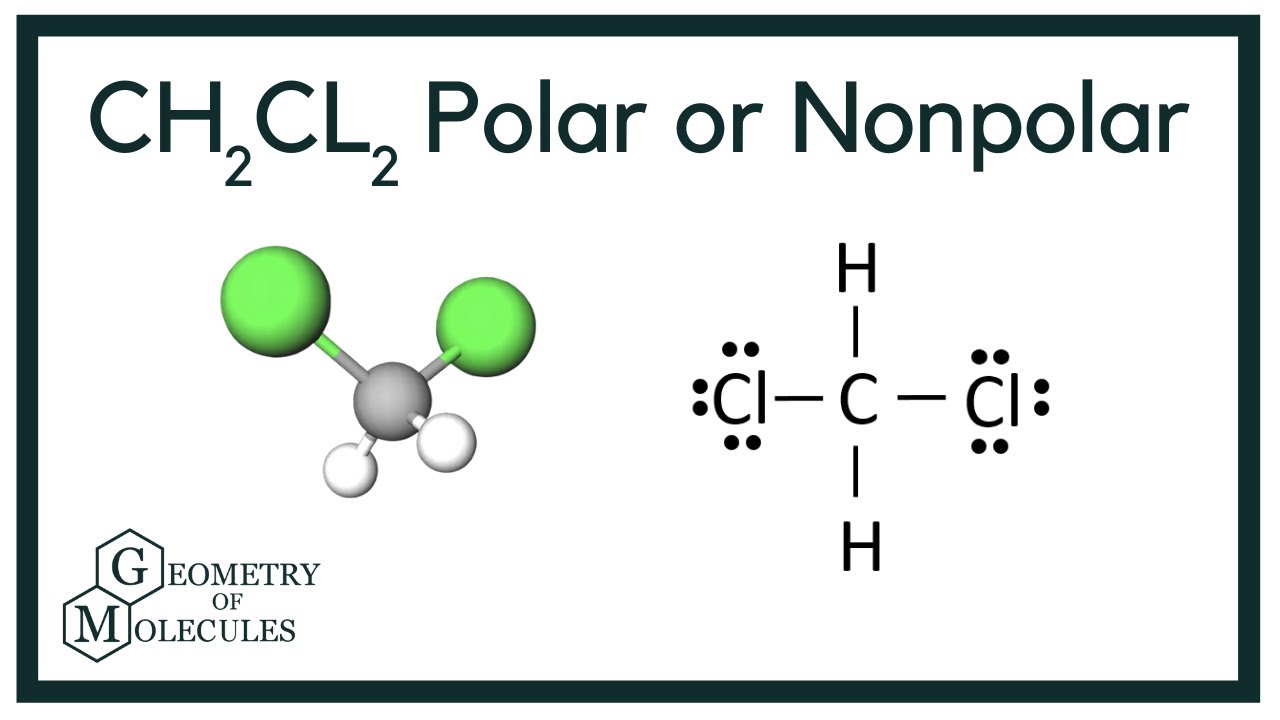
Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane
Database match 213 Exper.

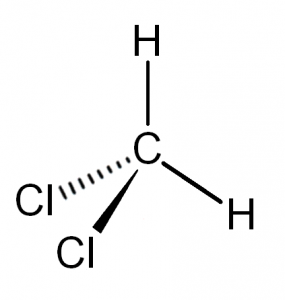
Ccl2h2 lewis structure. This is the Lewis structure for CH2Cl2. As the central atom has four bonded pairs and sp3 hybridization the shape of the molecule is tetrahedral. Rest all the non-bonding electrons are spread out in the structure.
8205 10 -24 cm 3. With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Carbon is less electronegative than Chlorine so itll go on the inside and Hydrogens always go on the outside.
Dichloromethane CH2Cl2 CID 6344 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Lewis Diagrams Made Easy How To Draw Dot Structures Play Download. The shape for ch2cl2 is tetrahedral.
Orbital Diagram of central atom After hybridization Bonding Scheme of central atom only Bonding Scheme of central atoms and peripheral atoms Electron Pair Geometry. The lewis structure drawing is really misleading because it makes you think that the chlorine atoms are directly opposite from each other on a 1D surface. So although the 4 dipole moments do point in the same direction they are not equivalent and thus do not cancel making the overall molecule polar.
Lewis Structure Orbital Diagram of central atom Before hybridization Bond Angle. The Lewis structure for CCl2H2 C C l 2 H 2 is shown below. Log Kow KOWWIN v167 estimate 212 Log Kow Exper.
A stepbystep explanation of how to draw the ccl2h2 lewis dot structure. To detemrine the structure the valence electrons should be calculates. Learn to draw lewis structures for molecular compounds topics.
CH2Cl2 Lewis structure For understanding the properties and structure of any chemical compounds including organic ones its lewis structure is of the utmost importance. On your lewis structure indicate any polar covalent bonds and the overall molecular polarity. Since H and Cl have different electronegativities the C-H and C-Cl dipole moments are also different.
How to draw lewis dot structures for molecules. The valence electrons. Predicted data is generated using the US Environmental Protection Agencys EPISuite.
Is the molecule Polar. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. For this compound the Carbon atom in the central position and rest all the Chlorine atoms are placed around it.
Dichlorodifluoromethane CCl2F2 CID 6391 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Molecule Lewis structure Cl 2 O CS 2 HCN b Lewis structures for TWO molecules are given below. Note that Hydrogen only needs two valence electrons to have a full outer shell.
We have a total of 20 valence electrons for CH2Cl2. Complete with formal charges show all resonance structures. The electronic configuration of Br is Ar3d10 4s2 4p5 A r 3 d.
AS 91164 current AS 90308 expired - Lewis Structures shapes of molecules 2004 2012 No Brain Too Small CHEMISTRY QUESTION 20081 a Draw a Lewis structure electron dot diagram for each of the following molecules. Lewis dot structures for covalent compounds part 1 this awesome video shows how to draw compounds. The Br belongs from the halogens family ie Group 17 thus the valence electrons present in Br is 7.
Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. Put our Hydrogens here and then our Chlorines. To draw the Lewis dot structure write the symbol for krypton Kr and then place two dots on the top bottom and two sides for a total of eight electrons.
In CCl2H2 the central C atom is attached to 2 H atoms and 2 Cl atoms. Dichlorodifluoromethane R-12 is a colorless gas usually sold under the brand name Freon-12 and a chlorofluorocarbon halomethane CFC used as a refrigerant and aerosol spray propellantComplying with the Montreal Protocol its manufacture was banned in developed countries non-article 5 countries in 1996 and developing countries article 5 countries in 2010 out of concerns about its. Drawing the Lewis Structure for C 2 H 2 Ethyne or Acetylene For C 2 H 2 you have a total of 10 valence electrons to work with.
Ccl2h2 Lewis Structure How To Draw The Lewis Structure For Ccl2h2 Youtube
Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane
Ch2 Ci2 Lewis Structure Novocom Top
Ch2cl2 Lewis Structure How To Draw The Lewis Structure For Ch2cl2 Dichloromethane Youtube
Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane
Ch2cl2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Ch2cl2 Lewis Structure How To Draw The Lewis Structure For Ch2cl2 Dichloromethane Youtube
Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane
Lewis Structure For Dichloromethane Novocom Top