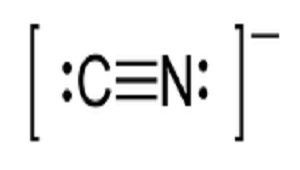Write the Lewis structure of glycine when this amino acid is dissolved in water. Carbon consist 4 valence electrons and nitrogen consist 5 valence electrons.
Cyanide Ion Lewis Structure - If you're searching for picture and video information linked to the key word you have come to pay a visit to the ideal blog. Our site gives you hints for seeing the highest quality video and picture content, search and find more informative video articles and graphics that fit your interests. includes one of tens of thousands of video collections from various sources, especially Youtube, therefore we recommend this video that you view. This site is for them to stop by this site.
Question 72701 Socratic
HCN Lewis Structure Molecular Geometry Shape and Polarity.

Cyanide ion lewis structure. C N X. CN Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. The most dangerous cyanides are hydrogen cyanide HCN and salts derived from it such as potassium cyanide KCN and sodium cyanide NaCN among others.
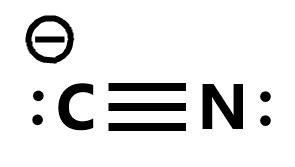
Write the Lewis structures of the ions that form when glycine is dissolved in 1 M HCl and in 1 M KOH. This molecule is a conjugate base for Hydrogen Cyanide. The typical Lewis structure for cyanide anion is C N.
Cyanide is a polyatomic ion with the formula CN -1. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It is lethal for every living thing and smells like bitter almonds.
Lewis diagram of the cyanide ion CN We can draw Lewis structures for polyatomic ions ions containing multiple atoms using the same stepwise procedure as for neutral molecules. Im not sure how to make ASCII Lewis structures. H C N.
Cyanide is a chemical compound with the chemical formula CN ion. Cyanide is a pseudohalide anion that is the conjugate base of hydrogen cyanide. Hydrogen Cyanide is a colorless flammable and poisonous chemical liquid.
Represented by the chemical formula HCN is one of those molecules that has an interesting Lewis structure. Cyanide ion is a chemical compound made up of one Carbon atom and one nitrogen atom with the chemical formula of CN-. X C N.
The carbon centre is associated with 7 electrons and is therefore a formal anion. The formula for the cyanide ion is C N X. In order to make sure the outer shell of the Nitrogen atoms are full you will need to form a triple bond in this Lewis structure between the Nitrogen and Carbon atoms.
Certainly in the parent hydrogen cyanide the acidic hydrogen is bound to carbon ie. Hi this is Dr. In this article we will study the Cyanide CN lewis structure or electron dot structure molecular orbital diagram MO its bond order formal charges and hybridization.
This group is also known as the cyano group. It carries a charge of -1 and is a conjugate base of hydrogen cyanide HCN. Many cyanide-containing compounds are highly toxic but some are not.
Also identify the sigma and pi bonds present in the structure and any non-bonding electrons in the molecule. There are two obvious ways to build the Lewis structure. We first count the number of electrons on the C atom.
The Lewis structure of cyanide ion eqleft rmCrmNrm - right eq is shown as follows. For the CN - Lewis structure there are a total of 10 valence electrons available. These cyanide compounds are poisonous in nature and are often referred to as Cyanide anion or Cyanide ion.
The Lewis structure for CN- has a total of ten valence electrons. This liquid is used in electroplating mining and as a precursor for several compounds. Chemists often represent ligands as spheres for simplicity even though the sphere sometimes has three-dimensional.
It is a conjugate base of a hydrogen cyanide. It is a member of the Cyano group. That gives us a total of ten valence electrons to work with.
It has a role as an EC 1931 cytochrome c oxidase inhibitor. Many compounds of cyanide are toxic and lethal upon exposure to it. There are 2 from the nonbonding pair of electrons and 3 from the 6 electrons in the triple bond for a total of 5.
See the Big List of Lewis Structures Transcript. Draw the Lewis dot structure for the cyanide ion CN- and identify the hybridization state of the carbon and nitrogen atoms. Lewis Dot of Cyanide Ion.
70 More Lewis Dot Structures. In this video well see how to construct the Lewis diagram of the cyanide ion. I hope that was clear.
In a titration of cyanide ion 2872 mL of 00100 M AgNO 3 is added before precipitation begins. Click to see full answer Keeping this in view what is the correct Lewis structure for CN. Lets illustrate these rules by calculating the formal charges on the C and N atoms in the cyanide ion CN- which has the Lewis structure.
Consist of a carbon atom triple bonded to a nitrogen atom. Cyanide can be found in the seeds or pits of apples apricots and peaches. C has 4 N has 5 and the negative sign indicates an additional valence electron.
Cn Lewis Structure Cyanide Youtube
What Are The Resonance Structures Of A Cyanide Ion Quora
Cn Lewis Structure How To Draw The Dot Structure For The Cn Youtube
Draw A Lewis Structure For The Cyanide Ion Including Lone Pairs And Formal Charges Study Com
Worked Example Lewis Diagram Of The Cyanide Ion Cn Ap Chemistry Khan Academy Youtube
How To Calculate The Formal Charges For Cn Cynide Ion Youtube
Cn Lewis Structure How To Draw The Dot Structure For The Cn Youtube
Cn Cyanide Ion Lewis Structure Molecular Geometry And Polarity Geometry Of Molecules
Cn Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist