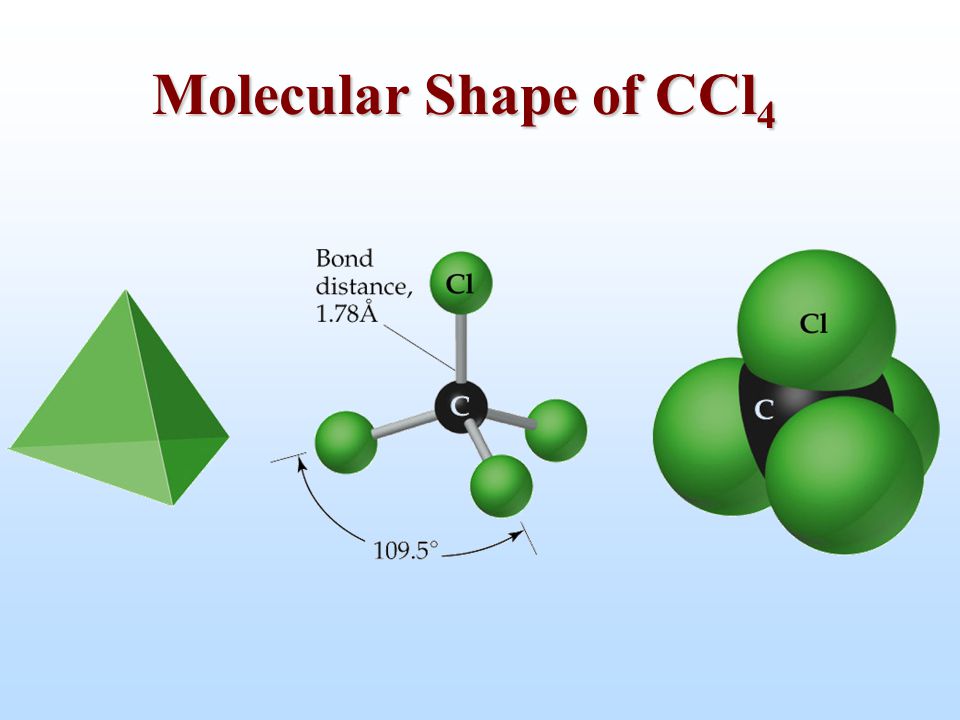
A quick explanation of the molecular geometry of ccl4 including a description of the ccl4 bond angleslooking at the ccl4 lewis structure we can see that. In the carbon tetrachloride molecule four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds.
Molecular Geometry Ccl4 - If you're looking for picture and video information related to the key word you have come to visit the right site. Our website provides you with suggestions for viewing the maximum quality video and image content, hunt and find more enlightening video articles and graphics that match your interests. comprises one of thousands of movie collections from several sources, especially Youtube, so we recommend this movie that you see. You can also bring about supporting this website by sharing videos and images that you like on this blog on your social media accounts such as Facebook and Instagram or tell your closest friends share your experiences about the simplicity of access to downloads and the information you get on this website. This blog is for them to visit this website.
Chapter 9 Molecular Geometry And Bonding Theories Ppt Video Online Download
Determine the electron geometry and molecular shape of this molecule.
Molecular geometry ccl4. See full answer below. Carbon is bonded to 4 Chlorine atoms. Determine the number of lone pairs on the central atom from the lewis structure.
Therefore CCl 4 is nonpolar. Bent Linear Tetrahedral Trigonal Pyramidal Not Enough Information. Because of this symmetric geometry CCl 4 is non-polar.
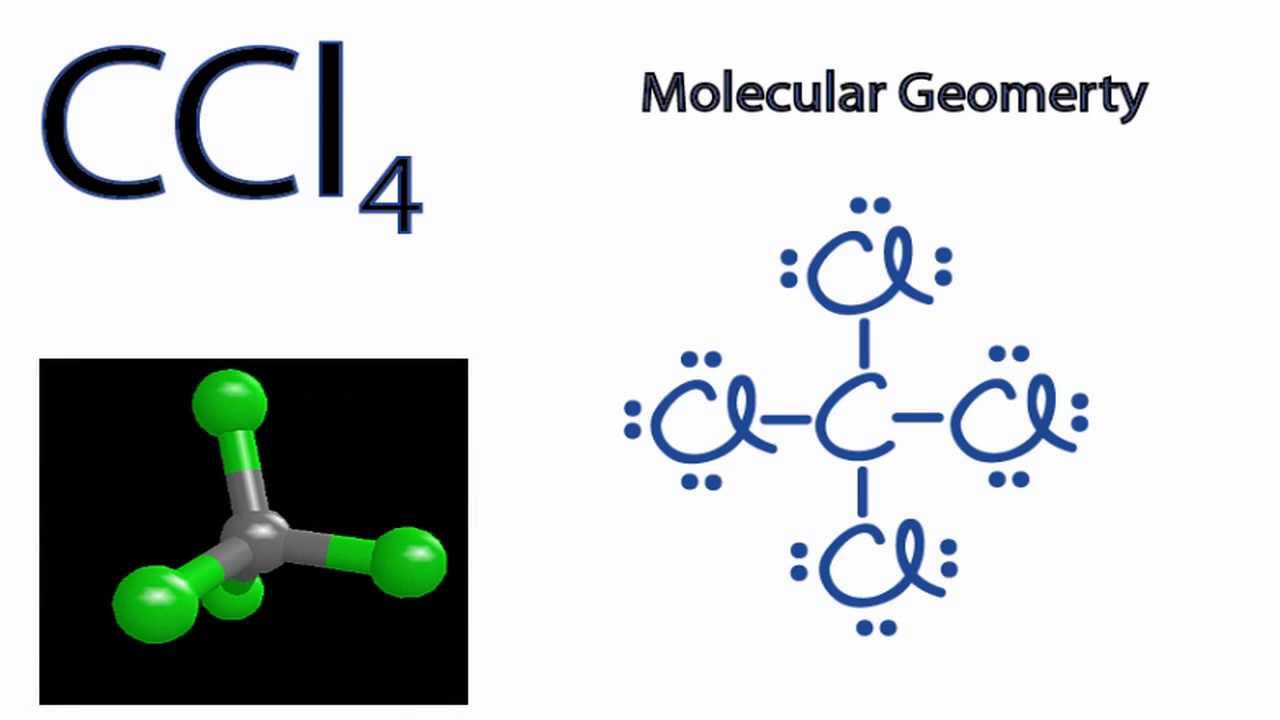
Four lines in the structure represent four bonds while dots around the Chlorine atom represent valence electrons. Four bonded pairs make the geometry of CCl4 to be Tetrahedral. This tells you the geometry that the molecule is based on.
CCl4 has a tetrahedral geometry with bond angles of 1095. Determine the shape of the. Hybridization is vital to understand the molecular geometry of the compound.
What Is The Molecular Geometry Of CCl4. Each Chlorine atom has six valence electrons after the bonds are formed. When the electron groups are all bond pairs they are named exactly like the electron-group geometryDetermine the electron geometry for each molecule CF4 NF3 OF2 H2S.
The possible molecular shapes are. Is this molecule polar or. Calculate the total number of valence electrons present.
As carbon with four bonded pairs and Sp³ hybridization in a central position and chlorine spread evenly around it all sides. Carbon tetrachloride CCl4 CID 5943 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. For this we need to do the following steps.
Molecular geometry on the other hand depends on not only on the number of electron groups but also on the number of lone pairs. CCl4 has 4 electron domains and is therefore based on tetrahedral. To do so we first need to draw a Lewis structure for CCl4.
The molecular geometry of CCl4 is Tetrahedral and its electron geometry is also tetrahedral with a bond angle of 1095º. The molecular geometry of CCl 4 is tetrahedral with symmetric charge distribution around the central atom. Here is how we can find out the geometry of CCl4.
The central atom of this compound is Carbon. Methane gas has the same structure making carbon tetrachloride a halomethane. Vsepr geometry the lewis structure shows that there are four electron regions about the central carbon atom.
CH3F is a polar molecule because the dipole between the C-H and C-F bonds are differents thus besides the symmetrical geometry the dipole bonds are not canceled. Draw the Lewis structure for the molecule. Draw the Lewis dot structure for CCl4.
Carbon Tetrachloride on Wikipedia. The Carbon atom takes a central place and the rest Chlorine atoms are placed around it. So there are a total of 24 non-bonding or 12 lone pairs of electrons in CCl4.
CO2 and CCl4 are both nonpolar because of the 3D geometry of the molecule. Each individual bond is polar but both molecules have symmetrical geometry so the dipole bonds are canceled. CCl 4 s molecular geometry is tetrahedral which means that its a triangular pyramid with four faces and vertex corners and six straight lines.
The molecular geometry of the given molecules is shown below. CCl4 has no lone pairs on the central carbon and is therefore of tetrahedral geometry. Determine the central atom in this molecule.
Ccl4 Molecular Geometry Shape And Bond Angles دیدئو Dideo
Ccl4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Molecular Geometry Of Ccl4 Draw Its Vsepr And Lewis Structure
Ccl4 Molecular Geometry Shape And Bond Angles Youtube
Is Ccl4 Polar Or Non Polar Carbon Tetrachloride Youtube
What Is The Molecular Geometry Of Ccl4 Draw Its Vsepr And Lewis Structure
Use The Vsepr Theory To Predict The Shape Of Carbon Tetrachloride Ccl4 A Tetrahedral B Bent C Trigonal Pyramidal D Trigonal Planar Study Com
Ccl4 Molecular Geometry Lewis Structure Hybridization And Everything
Ccl4 Lewis Structure Molecular Geometry Polar Or Non Polar Bond Angle