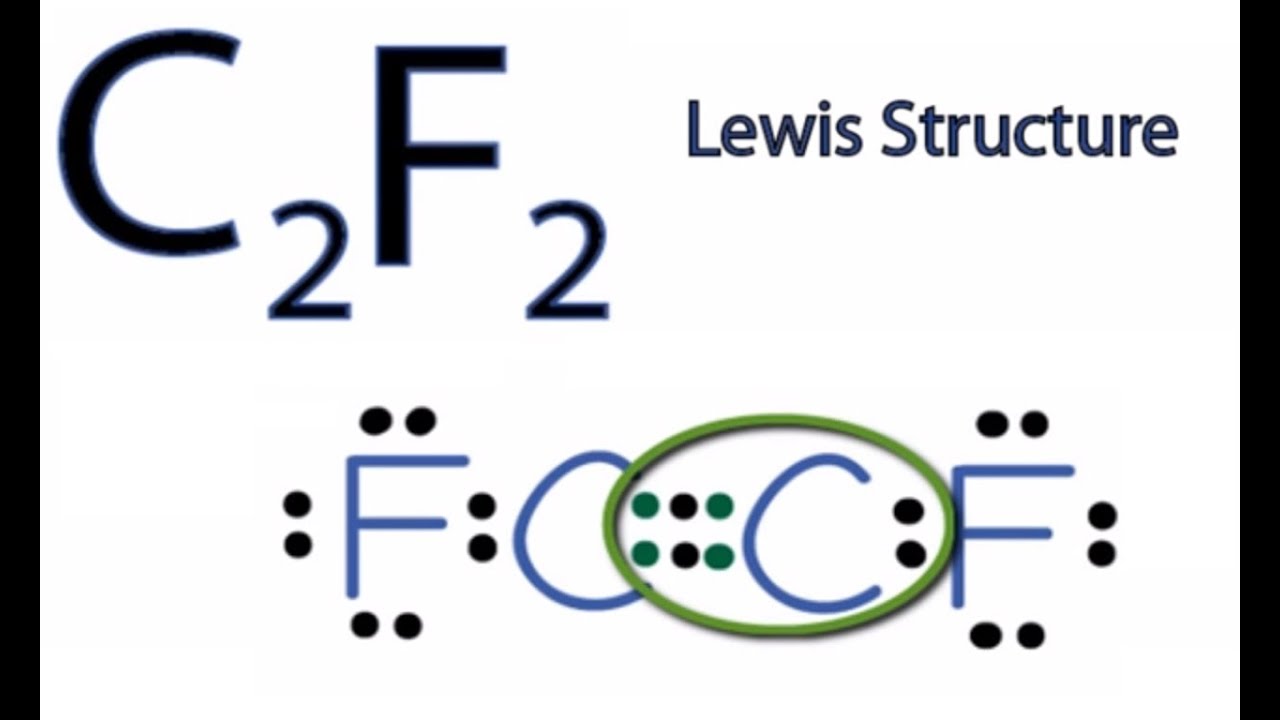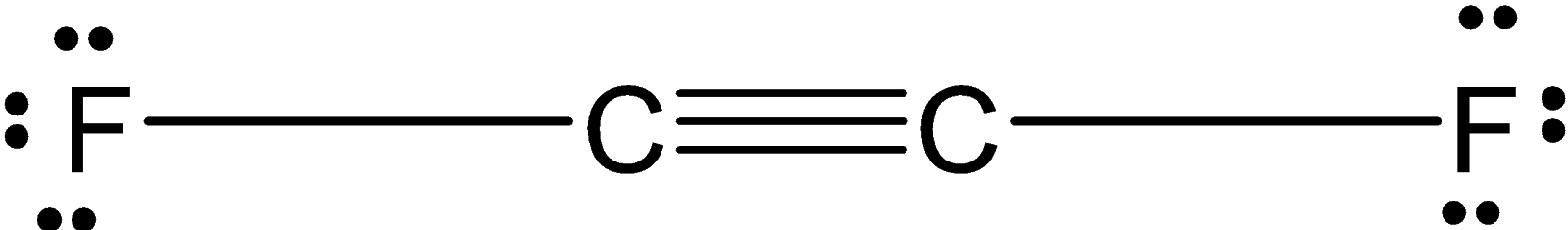This is a linear molecule containing two polar carbon-oxygen double bonds. Using Equation 851 the formal charge on the nitrogen atom is therefore.
Lewis Dot Structure C2f2 - If you're searching for video and picture information linked to the key word you've come to pay a visit to the right blog. Our website gives you hints for viewing the maximum quality video and picture content, hunt and find more enlightening video articles and graphics that match your interests. comprises one of thousands of movie collections from several sources, particularly Youtube, so we recommend this movie that you see. This site is for them to visit this website.
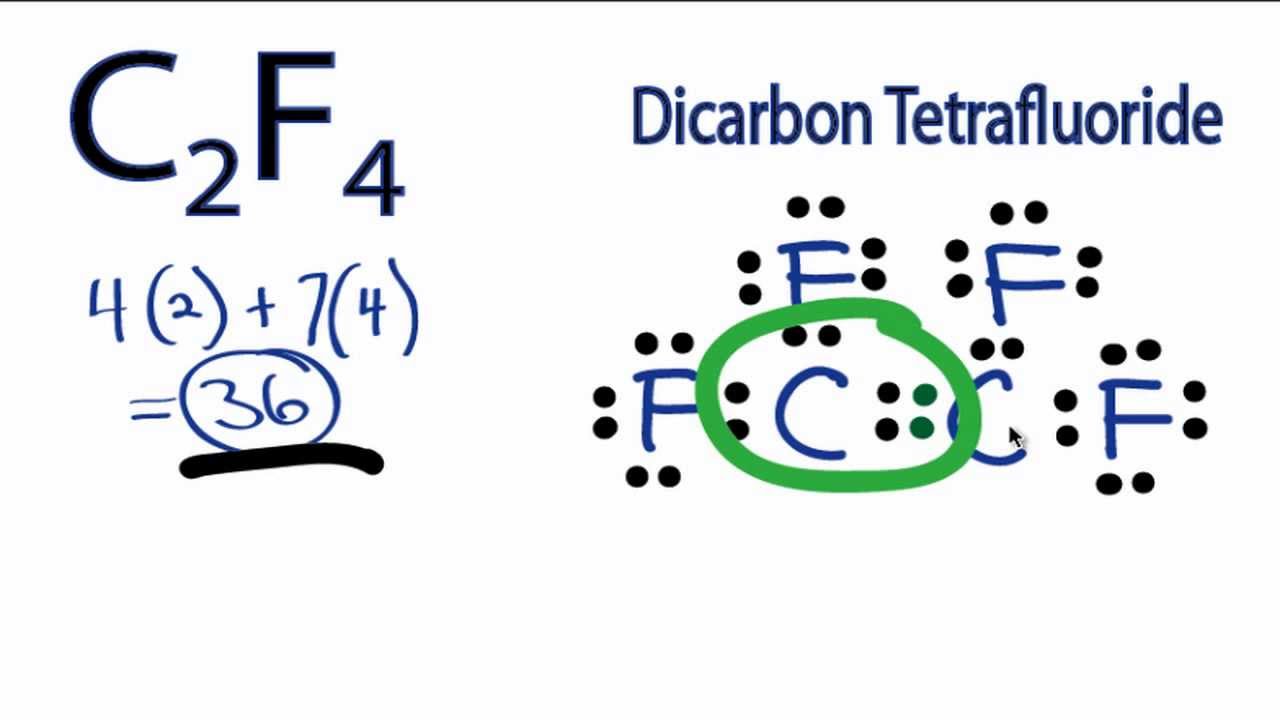
C2f4 Lewis Structure How To Draw The Lewis Structure For C2f4 Youtube
On the periodic table Carbon is in group 4 it has 4 valence electrons.
Lewis dot structure c2f2. Of atoms bonded to central atom atom 7. Therefore it is nonpolar and relatively unreactive. Where the metal loses electrons and the non-metal gains electrons creating charged ions.
The lewis dot structure of CO2 gives it some unique properties. Steric Number of central 4. 1043 f o r m a l c h a r g e N 5 0 8 2 0.
Complete the Lewis dot structure for C 2 F 6 which contains a C - C bond. The F 2 Lewis structure is similar to Br 2 Cl 2 and I 2 since F Br Cl and I are all in Group 7 and have 7 valence electrons. C2F2 F-C-C-F 1.
For example consider the Lewis dot structure for carbon dioxide. Yet More Lewis Structures Answers. The Lewis electron structure for the NH 4 ion is as follows.
Neutral Compounds Practice Problems. Calculate the formal charge of each individual atom and. Well also explore polyatomic ions and how to draw Lewis dot structures for them.
Put two canbon atoms in the center side by sidePut one fluorine on each carbon atom. Lines represent shared pairs of bonded electrons. Usually used to represent the bonding in these compounds.
Difluoroacetylene C2F2 CID 136491 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. However since the polar bonds are pointing exactly 180 away from each other the bond polarities cancel out and the molecule is nonpolar. Use information from step 4 and 5 to draw the lewis structure.
This lesson defines Lewis dot structures and explains how to draw them for molecules in step-by-step detail. The nitrogen atom shares four bonding pairs of electrons and a neutral nitrogen atom has five valence electrons. Draw the Lewis structures for the following molecules.
Since there are no lone pairs on the atom it is a linear structure which makes the charges cancel it. Hydrogen group 1 but we have 2 Hydrogens. Molecular compounds containing covalent or polar covalent bonds are more complex.
CHBrCl2 C2 2-NCl3N3 -N2F2C2F2. For those of you that enjoy such things some more Lewis structures to draw. Lets do the Lewis structure for CH2F2.
Alternatively a dot method can be used to draw the lewis structure of C 2 F 2 Calculate the total valence electrons in the molecule. Fluorine 7 valence electrons we have 2 of those as well for a total of 20 valence electrons. Calculate the formal charges for the atoms in the molecule S3O6 2-A chemistry student found that there are three possible ways to write the Lewis structures of nitrosyl fluoride NOF.
Lewis dot structure of C 2 F 2. Lewis electron structures give no information about molecular geometry the arrangement of bonded atoms in a molecule or polyatomic ion which is crucial to understanding the chemistry of a molecule. Hybridization of central atom Page 4 of 6 VSEPR Theory and the Shapes of Molecules.
By signing up youll get thousands of step-by-step solutions to your homework questions. Electrons are shown as dots or for bonding electrons as a line between the two atoms. How to Draw the Lewis Dot Structure for F2.
Learn this topic by watching Lewis Dot Structures. The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. Difluoromethane CH2F2 CID 6345 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
Draw the Lewis structure for C2F2. Of lone electron pairs on central atom 6. Neutral Compounds Concept Videos All Chemistry Practice Problems Lewis Dot Structures.
3 C2H 5OH ethanol 4 N2F4. With BrF 2 there are an odd number of valence electrons 21 total. Well put the Carbon at the center and then Hydrogens always go on the outside.
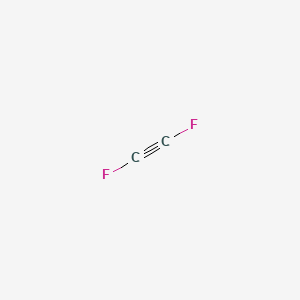
What Does The Ball And Stick Model Look Like For C2f2 June 30th 2015 - Answer To What Does The Ball And Stick Model Look Like For C2f2 Media Portfolio Pearson Education April 18th 2018 - This ball and stick model shows that water has its three atoms and two lone pairs arranged in a tetrahedron Notes Lewis dot structure for a PCl3 molecule.
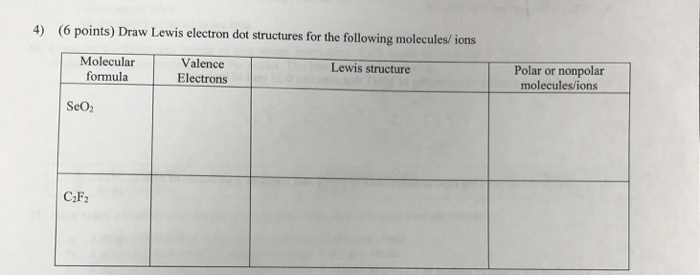
4 6 Points Draw Lewis Electron Dot Structures For Chegg Com
Lewis Structure Of C2f2 Biochemhelp
C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 Youtube
Which Substance Contains A Triple Bond 1 C2f2 2 H2co 3 Clutch Prep
C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 By Wayne Breslyn
C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 Youtube
C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 Youtube
The Shape Of O2f2is Similar To A C2f2 B O2h2 C H2f2 Class 11 Chemistry Jee Main
Difluoroacetylene C2f2 Pubchem