This was seen in the balloon example we used in class. And Te have hybridization s p 3 d 2 in T e O 6 6 P have hybridization s p 3 d 2 in P F 6 Si have hybridization s p 3 d 2 in S i F 6.
Teo6 6 Shape - If you're searching for picture and video information related to the key word you've come to pay a visit to the right site. Our site provides you with hints for seeing the highest quality video and image content, search and find more informative video articles and graphics that fit your interests. comprises one of tens of thousands of video collections from various sources, especially Youtube, so we recommend this video for you to see. This site is for them to stop by this site.
Answer The Questions Below About The Shape Clutch Prep
What word or two-word phrase best describes the shape of the TeO66 anion.

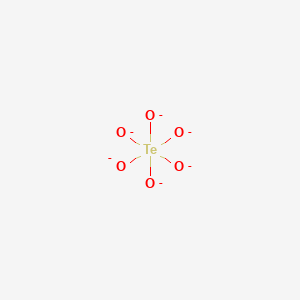
Teo6 6 shape. It is a conjugate base of an orthotellurate 5-. When four balloons of the same size are tied together the natural arrangement is as a tetrahedron. It reacts with strong bases to make tellurates.
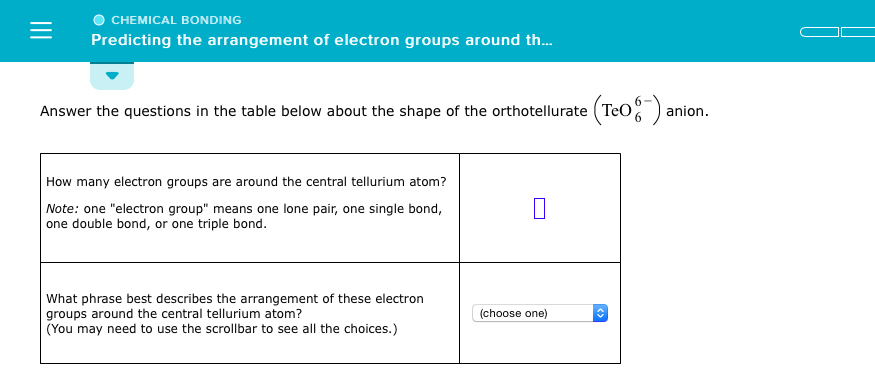
Answer the questions in the table below about the shape of the orthotellurate TeO66- anion. The molecular geometry and vibrational frequencies of TeF 6 have been calculated using different quantum chemical Hartree-Fock MP2 DFT theories in conjunction with various basis set combinations including relativistic effective core potentials supplemented with polarised double- and triple-zeta valence basis sets for Te and with a 6-31G 6-311G and Dunnings correlation consistent bases for F. An example of a molecule having an AX 6 designation is sulfur hexafluoride.
The molecular shape octahedral is shown below. Log Kow KOWWIN v167 estimate -067 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN v142. The Lewis structure is shown below.
How many electron groups are around the central tellurium atom. Here Xe have hybridization s p 3 d 2. 85074 Adapted Stein Brown method Melting Pt deg C.
First state the central atom. Property Name Property Value Reference. Get 1 free homework help answer.
Answer the questions in the table below a the shape of the orthotellurate TeO6-_6 anion. The crystal structure of Pb 6 Co 9 TeO 6 5 can be described in terms of 001 layers A at z 025 and B at z 0 that stack alternately along 100 Fig. For unlimited access to Homework Help a Homework subscription is required.
It is a powerful oxidizing agents. Orthotellurate 6- is an orthotellurate ion. It can be dehydrated to make tellurium trioxide.
- Molecules having a central atom surrounded by six atoms and no lone pairs of electrons have an AX 6 designations. In layer A TeO 6 and CoO 6 octahedra share edges with 16 of the octahedral holes at the 2c and 2d positions both with site symmetry 32 not. An example of this is sulfur hexafluoride SF 6.
Raumgruppe P2 1 a. What is the Lewis structure of TeO 6 6-. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count.
A Te then state bonded atoms 6 O xy View the full answer Transcribed image text. Charakteristisch für die Struktur sind oktaederförmige Gruppen TeO 6Die Struktur wird. A quick explanation of the molecular geometry of ClO4 - Perchlorate ion including a description of the ClO4 - bond anglesLooking at the ClO4- Lewis struct.
Molecules having an AX 6 designation have an octahedral geometry and bond angles of 90o. TeF6 or Tellurium Hexafluoride has a very simple Lewis structure. What phrase best describes the arrangement of these electron groups around the central tellurium atom.
What is the angle between the tellurium-oxygen bonds. Telluric acid is a weak acid. Cu10Zn3TeO66 crystallizes in the triclinic P1 space group.
β 12274. The geometry should be square pyramidal on top and bottom. The shape of this molecule is a result of the electrons in the four bonds positioning themselves so as to minimize the repulsive effects.
Predicted data is generated using the US Environmental Protection Agencys EPISuite. It contains the TeO 6 6-form of the tellurate ion. Answer the questions below about the shape of the orthotellurate TeO 6 6- anion.
A 7255 pm b 9833 pm c 6667 pm. Neu dargestellt wurde aus den binären Oxiden in Form farbloser Einkristalle K 4 Na 2 TeO 6K 2 ONa 2 OTeO 3 211 700C 28 Tage. 277E-027 Modified Grain.
Xe have oxidation no 8 in X e O 6 4. Log Octanol-Water Partition Coef SRC. 4 Polymeric tellurate ions edit.
In the first Cu3 site Cu3 is bonded to six O2- atoms to form distorted CuO6 octahedra that share a cornercorner with one CuO6 octahedra corners with two TeO6 octahedra a cornercorner with one ZnO4. Its chemical formula is H 6 TeO 6. 10 made up of two edge-sharing TeO 6 as in Li 4 TeO 5 and Ag 4 TeO 5 or corner-sharing TeO 6 octahedra as in Hg 2 TeO 5.
There are ten inequivalent Cu3 sites. R 94 R w 71 für 1374 symmetrieunabhängige Reflexe Ag K ga. A compound with 6 atoms surrounding the central atoms will have an octahedral geometric shape and an octahedral molecular shape.
Boiling Pt deg C. Place The The atom in the center single bonded to 6 F atoms. Already have an account.
Computed by Cactvs 34611 PubChem release 20190618. The structure is three-dimensional. 34984 Mean or Weighted MP VPmm Hg25 deg C.
What is the angle between the tellurium-oxygen bonds. If there is more than one angle pick the smallest. It contains hydrogen and tellurate ions.
All of the bond lengths and angles 90 o are identical in this structure.
Answer The Questions In The Table Below About The Shape Of T Clutch Prep
Answer The Questions In The Table Below A The Shape Chegg Com
L Sup 6 Sup Tellanehexaylhexaoxidanide O6te Chemspider
Answer The Questions In The Table Below About The Shape Of T Clutch Prep
Scl2 Bond Angle Shefalitayal
Orthotellurate 6 O6te 6 Pubchem
Answer The Questions Below About The Shape Clutch Prep
Answer The Questions Below About The Shape Clutch Prep
Answer The Questions Below About The Shape Clutch Prep