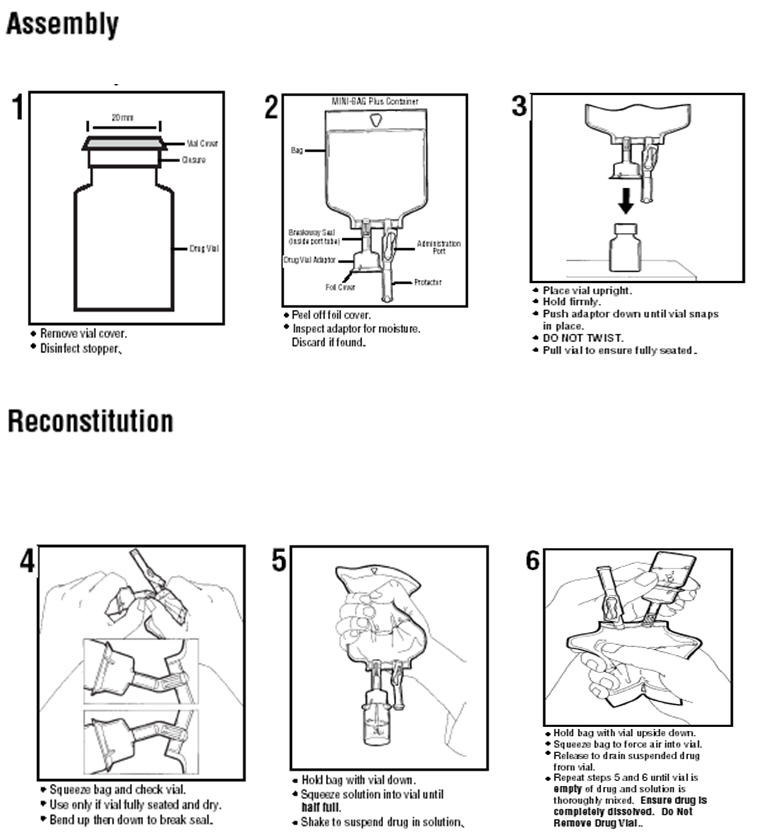
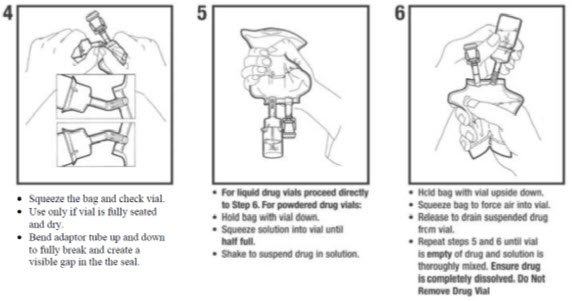
For use only with single dose powdered and liquid up to 10 mL drug vials with standard 20 mm closures. It allows for drug admixture after connection to a single dose powder or liquid up to 10 mL drug vial having a 20 mm closure.
Baxter Mini Bag Plus Stability - If you're searching for picture and video information linked to the keyword you've come to visit the right site. Our website provides you with hints for seeing the maximum quality video and picture content, hunt and locate more informative video content and images that fit your interests. comprises one of thousands of video collections from various sources, particularly Youtube, so we recommend this movie for you to see. This site is for them to visit this website.
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2015 020178s013lbl Pdf
7 out of 10 healthcare professionals rate Mini-Bag Plus containers as very easy to use 1.
Baxter mini bag plus stability. 50 and 100 mL bags. 100 mL containers docked with the following drugs. Use within specified time for drug stability.
Cefazolin 1 g cefuroxime Zinacef 750 mg ceftriaxone Rocephin 1 g aztreonam Azactam 1 g piperacillin and tazobactam Zosyn 3375 g. DOPamine Hydrochloride in 5 Dextrose Injection 1600 mcgmL in 500 mL VIAFLEX Plastic Container. 09 Sodium Chloride Injection USP 50 mL MINI-BAG Plus Container.
Mini-Bag Plus ADD-Vantage Vial-Mate Adaptors Add-a-Vial. The CDC stipulates that the. To report product quality issues please contact Baxter Product Surveil lance at 1-800-437-5176.
Solutions of Teflaro in concentrations ranging from 4 to 12 mgmL in Baxter Mini-Bag Plus containers with 09 Sodium Chloride Injection may be stored for up to 6 hours at room temperature or for up to 24 hours at 2C to 8C 36F to 46F. DOPamine Hydrochloride in 5 Dextrose Injection 800 mcgmL in 250 mL VIAFLEX Plastic Container. 43 faster than manual mixing 1.
A Mini-Bag Plus Container allows for medication mixture to happen on-site. In MINI-BAG Plus Container VIAFLEX Plastic Container DESCRIPTION 09 Sodium Chloride Injection USP in the MINI-BAG Plus Container is a sterile nonpyrogenic solution for intravenous administration after admixture with a single dose powdered drug. Stability testing in the Baxter Mini-Bag Plus has solely been conducted on 50 mL and 100 mL containers 09 Sodium.
Baxter IV solutions 150ml in plastic overwrap can be warmed at max 104 F for up to 14 days. DOPamine Hydrochloride in 5 Dextrose Injection 3200 mcgmL in 250 mL VIAFLEX Plastic Container. The preparation involves not more than 3 different sterile products.
Any unused starting component from a single-dose container must be discarded after preparation for the individual patient is complete. The drugs eg FDA-approved labeling stability studies. The nominal pH is.
Sodium Chloride Injection USP in VIAFLEX Plastic Container DESCRIPTION Sodium Chloride Injection USP is a sterile nonpyrogenic solution for fluid and. A breakaway seal in the tube between the vial adaptor and the container is broken to allow transfer of the diluent into the vial and reconstitution of the drug. The MINI-BAG Plus Container is a standard diluent container with an integral drug vial adaptor.
Products are FDA-Approved - follow manufacturers instructions for handling and storing. It allows for drug admixture after connection to a single dose powdered or liquid up to 10 mL drug vial having a 20 mm closure. If remove at 14 days can use until manufacturer expiration.
Stability in Baxter Mini-Bag Plus. Only for Single Dose Powdered Drug Vials with 20 mm Closures Use Aseptic Technique To Order Baxter Products call 1-888-229-0001 Assembly. Presented as an Exhibitor Theater at the 42nd ASHP Midyear Clinical Meeting December 3 2007.
It contains no antimicrobial agents. Must note on product that cannot be rewarmed need process or discard. To place an order please contact Baxters Center for Service by calling 1 -888-229-0001.
Follow these simple instructions to mix the medication prior to the infusion. Single-dose containers must not be used for more than 1 patient. Compatible with powder and liquid up to 10 mL single-dose vials with 20 mm closure.
Revisions to USP Chapter Examining Sterile Compounding Practices. To report adverse events associated with these imported products please call Baxter. ADD-Vantage MINI-BAG PLUS addEASE Attachingactivating these not considered compounding Acceptable for nursing to attach and activate Use manufacturers instructions for storage and stability The CDC advised on a more conservative approach to further safeguard patients.
Mannitol package inserts recommend room. 30 days from date diluent removed from overwrap. Product information last updated Jun-23-2021.
Administer medication according to directions. Minibag plus The Minibag Plus Container is a standard diluent container with an integral closed system transfer device CSTD drug vial adaptor. In-use ceftaroline fosamil infusion solution ranging from 4-mgmL to a maximum of 12-mgmL concentration in elastomeric home infusion systems prefilled with 09 sodium chloride injection or 5 dextrose and MINI-BAG Plus Containers prefilled with 09 sodium chloride injection were chemically stable for up to 24 hours refrigerated at 2C to 8C 36F to 46F and up to 6 hours at room.
0 9 Sodium Chloride Injection Usp In Mini Bag Plus Container
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2015 020178s013lbl Pdf
0 9 Sodium Chloride Injection Usp In Mini Bag Plus Container
Https Www Baxter Ca Sites G Files Ebysai1431 Files 2019 04 0 9 Sodium Chloride Injection Usp In Viaflex En Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2015 020178s013lbl Pdf
Dextrose Injection Mini Bag Fda Prescribing Information Side Effects And Uses
Https Www Baxter Ca Sites G Files Ebysai1431 Files 2019 04 0 9 Sodium Chloride Injection Usp In Viaflex En Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2015 020178s013lbl Pdf
Sodium Chloride Mini Bag Fda Prescribing Information Side Effects And Uses